Abstract

Introduction: Myelofibrosis (MF) is a serious and life-threatening myeloproliferative neoplasm that is characterized by stem cell-derived clonal myeloproliferation, bone marrow fibrosis, anemia and splenomegaly. The Janus kinase (JAK) pathway is the critical pathway in its pathogenesis. Ruxolitinib, a JAK1/2 inhibitor, was the first US Food and Drug Administration (FDA)-approved therapy for intermediate- and high-risk MF. However, there remains a high unmet need for alternative treatment options for patients who discontinue (41.1%-60.9% of patients discontinue after 3 months and 48.4%-73.0% after 6 months) (Fonseca E, et al. Blood 2013;122. Abstract 2833) or are no longer responding to ruxolitinib therapy (Bose P, Verstovsek S. Leuk Lymphoma 2020;61:1797-1809). The efficacy and safety of fedratinib, a JAK2 inhibitor approved by the FDA in 2019, was investigated post ruxolitinib in the single-arm trial JAKARTA-2 (NCT01523171) (Harrison CN, et al. Lancet Haematol 2017;4:e317-324; Harrison CN, et al. Am J Hematol 2020;95:594-603). New clinical evidence for treating a similar population with navitoclax plus ruxolitinib was presented at the American Society of Hematology Annual Meeting in 2020 (Pemmaraju N, et al. Blood 2020;136(suppl 1):49-50). The efficacy of fedratinib relative to navitoclax plus ruxolitinib in patients with MF previously treated with ruxolitinib has not yet been evaluated.
Objective: To explore the comparative efficacy of fedratinib versus navitoclax plus ruxolitinib in patients with MF previously treated with ruxolitinib for the 2 binary endpoints of ≥ 35% spleen volume reduction (SVR) from baseline to the end of cycle 6 (EOC6; 24 weeks) and ≥ 50% reduction in total symptom score (TSS) from baseline to the EOC6.
Methods: Evidence for fedratinib was informed by JAKARTA-2 patient-level data, and evidence for navitoclax plus ruxolitinib was informed by known reported evidence from the REFINE study (NCT03222609) (Pemmaraju N, et al. Blood 2020;136(suppl 1):49-50; Harrison CN, et al. J Clin Oncol 2019;37 (suppl 15). Abstract 7057). The suitability of these studies for indirect treatment comparison (ITC) was assessed by considering the comparability of study design, population, intervention, and outcomes. Given the lack of a common comparator in the identified studies, unanchored ITCs were performed for SVR using matching-adjusted indirect comparison (MAIC) and simulated treatment comparison (STC) methods. Univariable and multivariable regression models were used to identify potential prognostic factors to adjust for in the ITCs. Additionally, all reported Dynamic International Prognostic Scoring System (DIPSS)-Plus criteria were considered. Where sample size was too small, response rates (number of responders divided by total number of patients) were compared naively.
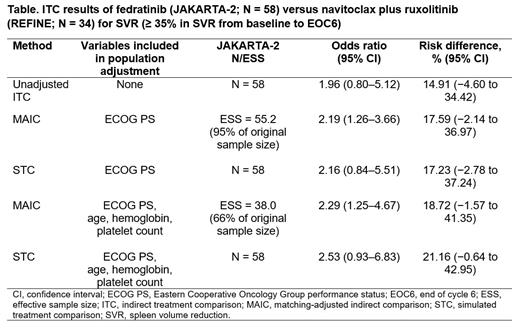
Results: A subgroup of 58 JAKARTA-2 patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1 and intermediate-2 or high-risk disease most closely aligned with the REFINE population was used in the analyses. Baseline mean platelet count was similar between subgroups. Across the analyses performed, results suggested fedratinib consistently increased the odds/risk of a spleen response compared with navitoclax plus ruxolitinib (Table). The MAIC, matching on ECOG PS, suggested that the odds of having an SVR for patients in the fedratinib group was 2.19 times (95% confidence interval [CI], 1.26 to 3.66) that of the navitoclax plus ruxolitinib group, and the risk of having an SVR for patients in the fedratinib group was 17.59% higher (95% CI, −2.14 to 36.97). The results from the MAIC that additionally matched on all possible DIPSS-Plus criteria (age, hemoglobin, and platelet count) were consistent. Results from the 2 methods (MAIC and STC) were also consistent. For TSS reduction, the sample size (N = 20) in REFINE was considered too small to perform a meaningful ITC; however, the absolute response rates for TSS reduction were similar across the 2 groups (29% [16/56] in the fedratinib group and 30% [6/20] in the navitoclax plus ruxolitinib group).
Conclusion: In the population of patients with MF previously treated with ruxolitinib, these analyses suggest treatment with fedratinib was associated with a greater proportion of patients achieving a spleen response compared with navitoclax plus ruxolitinib. Limited data were available for comparison of TSS.
Abraham: Bristol Myers Squibb: Current Employment. Liao: BMS: Consultancy. Chevli: Bristol-Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Smith: Sarah Smith is an employee of BresMed. BresMed received consultancy fees from BMS/Celgene for the reasearch in this abstract. She did not receive direct payment as a result of this work outside of her normal salary payments.: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract